as found by sugar,
chromatography, and PPO tests.
by The Lipids
Ryan Dorough, Allison Dobrovich, and Stephanie Hreha
Abstract
In order to determine how a particular diet affects the chemical composition of a cricket, we tested for the presence of carbohydrates, pigments, and enzymes in two different types of food, and in the crickets that were put on the two different diets. We believe that diet a cricket's diet will influence its chemical. The crickets were either fed a special food called "Cricket Total Bites", or they were fed dry oats for nutrients and potato for moisture. Various carbohydrate tests (Barfoed's, Selivanoff's, Bial's, and Iodine) were performed as well as paper chromatography to test for pigments, and enzymes tests for the presence of PPO. The sugar present in the cricket food turned out to be a monosaccharide, ketose, and hexose furanose. The cricket food's most abundant pigments were found to be chlorophyll a, xanthophyll, and no PPO was found. The same tests were used for all substances. The crickets on the cricket food diet included aldose and pyranose sugars, and no PPO. The potato and the oatmeal were found to contain disaccharide ketose and pentose furanose sugars, starch and no PPO or pigments. The crickets that ate this food showed sugars of an aldose and pyranose, and again were negative to PPO and pigments. These results suggest that there is no direct correlation between the foods tested and the cricket solutions that ate that food.
Discussion
The business of cricket farming is a multimillion dollar industry, and it is essential that farmers stay in demand by raising crickets that will be nutritious to the reptiles that eat them. Since crickets are used as an essential part of the diet of many reptiles, it is necessary that they have the proper nutrients to pass along to the reptiles that consume them. The company that produces the cricket food “Cricket Total Bites” claims that this food has the perfect combination of nutrients required by crickets (Pretty Pets Reptile Products, 2003). If “Cricket Total Bites” is in fact the most nutritious food then it would be to the farmers benefit to feed it to their crickets. However, this special food is relatively expensive.
The smallest jar sold of this food is $5.95 and was only enough to feed our 24 crickets for a total duration of three weeks. Providing this food for the millions of crickets that farmers raise would get to be very expensive and possibly not even result in any profit. An inexpensive supplement such as oatmeal and potatoes would greatly boost income in the industry if it does not change the nutrients present in the cricket, which is what we decided to determine. We believed that crickets fed a cheaper diet of oatmeal and potatoes would contain the same carbohydrates and enzymes as those which were fed the “perfect” diet of “Cricket Total Bites”. After performing a series of carbohydrate tests, a chromatography test for pigment, and tests on PPO, we found that a cricket’s diet has no significant impact on its overall chemical composition.
A reptile expert at the Preuss Animal House informed us that a cricket’s body will acclimate to a new diet around every four days, by that time everything in its old diet will have been digested and passed through the body. Before we purchased the crickets they had been fed the “Cricket Total Bites” cricket food, which is what we continued to feed a dozen crickets. We changed the diet of another dozen crickets to one of oatmeal and potatoes seven days before we performed our tests. By this time their digestive tract was already cleaned out, and the food they had previously eaten was no longer present in their bodies.
To see if a change in cricket food resulted in a change of carbohydrates within the cricket’s body we performed sugar testing. The first series of tests performed identified the properties of any reducing sugars found in a given substance. In performing the Barfoed’s, Selivanoff’s, Bial’s, and Iodine tests for reducing sugars, we were able to predict the structures of the carbohydrates found in the five tested solutions: cricket that ate cricket food, cricket food, cricket that ate potatoes and oats, potatoes, and oats. Solutions were made of each of these items by adding water and blending them individually.
The first test performed, Barfoed’s test, determined whether the sugars present are mono-, di-, or polysaccharides by testing how long a given solution takes to reduce copper ions in the presence of Barfoed’s solution and while being boiled. Solutions containing monosaccharides will react during two minutes of boiling, changing the solution to a rusty color, but di- and polysaccharides will not react within that time (Maleszewski et al., 2003). According to Figure 1, the cricket food is the only solution that showed a presence of monosaccharides. This means that any sugars present in the other solution are probably di- or polysaccharides, which could be determined by further testing.
Selivanoff’s test is used to determine the presence of aldoses and ketoses in a solution. When Selivanoff’s reagent is added, a solution with ketoses will turn a red color within one minute of boiling and those with aldoses will take a few minutes to react. If the solution contains only a monosaccharide ketose, it will react immediately. If it contains a disaccharide ketose, then it will react after very close to a minute (Maleszewski et. al., 2003). With a color change to red immediately, we found that the cricket food contains a monosaccharide ketose. The potato and the oatmeal solutions, which turned red in approximately one minute, contained a disaccharide ketose. Both cricket solutions, taking around two minutes for only a slight color change, showed the presence of an aldose (Figure 2).
The third carbohydrate test performed, Bial’s test, identifies the presence of a furanose ring. With the addition of Bial’s reagent, the solutions containing furanoses will form a green solution when boiled for five minutes. Solutions with pentose-furanoses will turn an olive-green color under the same circumstances. A hexose (or higher)-furanose turns a muddy brown color with the addition of Bial’s reagent. If no color change occurs, the sugar is a pyranose (Maleszewski et. al., 2003). Remaining its original color, neither cricket solution was shown to contain a furanose ring; meanignt he sugar is a pyranose. The cricket food showed the presence of a hexose (or higer)- furanose by turning a greenish brown color. Both the potato and the oatmeal turned green in solution showing presence of a furanose (Figure 3).
The final carbohydrate test we performed was the Iodine test. The Iodine test shows the presence of starch when a solution turns a bluish black color with the addition of the reagent IKI (Maleszewski et. al., 2003). The only solutions that showed a positive test for starch were the potato and oatmeal solutions, all other solutions remained a light yellow/brown color i.e. no change (Figure 4).
At the same time that we were performing these tests on our substances, we were also doing control testing. Each test which was performed on our substances was performed using the carbohydrates on pages 64-66 in the lab manual, as was done a week earlier (Maleszewski et. al., 2003). This way we could compare the results of the control to those which were received the previous week to ensure that the same results were found and there was nothing different about the new solutions. Our results do not show a clear relationship between the reducing sugars found in the crickets and the food that they consumed. We originally predicted that if different carbohydrates were found in the different food sources then different carbohydrates would be found in the two groups of crickets that ate that food. What we found was that the two groups of crickets contained the same sugars regardless of the food they ate.
When food enters a cricket’s body it passes through the foregut and into the midgut. Here the digestive enzymes are stored and they are released and start to digest the food. The carbohydrates and other parts of the food that have been broken down are absorbed into the body cavity (Breene III,2003), which explains why we did not detect them. The sugars that were in the different cricket foods did enter the bodies of the crickets, but when they did they were broken down and absorbed by the cricket. So the sugar tests that we performed did not show a correlation between sugars eaten and sugars present in the body (Figure 7). What sugars we found in the cricket may have been an altered form of those ingested, which could be determined through further testing. It is possible that we did not test for the right sugars, and that other tests would have shown a difference in the different groups of crickets. There is much more research that can be done on this topic using different and more advanced testing. Going on our results there is no evidence that the sugars consumed affect those present within the body.
In order to determine if a change in diet changed pigments inside the cricket’s body we moved on to our next test. The next test performed was the pigment identification test using paper chromatography, which determined which pigments were present in the crickets and their food. When a solution is dropped on a paper strip and the end of that strip is placed in a solvent, the solvent will travel up the strip carrying the different pigments with it. Each individual pigment that is present in the solution will be carried up a different distance depending on its solubility and will therefore be separated. When you divide the distance a pigment travels by the distance the solvent travels you get the Rf value (Maleszewski et. al., 2003). This value is useful in comparing the pigments in different solutions because when different trials are performed not all solvents will travel the same height, but the ratio of solution to solvent should always be the same.
The only solution in which we found pigments was the cricket food. This was not surprising since the cricket food was the only solution to have any color to it. The pigments that were found were xanthophyll (pale yellow, Rf value of .083), and chlorophyll a (blue-green, Rf value of .042). Neither cricket solution showed any pigment, which means the pigment from the cricket food was not carried over to the crickets. Again, there was a control used in this experiment. A paper chromatography test was also done on chlorophyll acetone solution using the exact procedure outlined in the lab manual which was also done a week earlier in lab (Maleszewski et. al., 2003). This way we could compare both results of the chlorophyll acetone solution from the different weeks to make sure that the solvent was working the same.
The chromatography test that we performed to determine the pigments present in the food and the crickets did support our hypothesis. We assumed that the pigments a cricket eats would not be shown in its body. This makes sense because we did not observe any change in the color of the cricket’s body as its diet changed.
The two pigments present in the cricket food were xanthophyll and chlorophyll a. The chemical structure of chlorophyll a is a magnesium atom surrounded by a large ring structure, and coming off of the ring structure is a long tail of isoprene units. The chemical structure of xanthophylls is made up of two hexane rings attached by isoprene units (Freeman, 2002). Once these pigments are digested in the body of an insect, they are not in their original form any longer. For example, the lutein that is contained in xanthophylls is used by the body as a nutrient that has been shown to reduce diseases such as heart disease. Now, the bonds holding their structures together have been broken and they are processed in the body, no longer working as pigments and giving color (Guo, last accessed 2-27-03). This is why we don’t see any color through a paper chromatography test, because it is no longer really a color.
To test if enzymes that are present in a cricket’s diet affect those that can be found within their bodies, we tested the presence of PPO, an enzyme that we knew was in potatoes due to previous testing. When catechol is added to a solution containing PPO, that solution will undergo a color change and turn into a pink/brown color (Maleszewski, 2003). When catechol was added to each of our five solutions the only one which resulted in a color change was the potato solution. When no results were found in the other solutions, we added more catechol to see if there would be a difference, but the potato was still the only one with a color change. A week before we performed this experiment, we had performed the same PPO test on a potato. Since a potato was one of the things that we were testing we knew what there should be a color change to a pinkish brown color, when catechol was added, if the catechol was working the same way it was the previous week. This was the only control that we needed in this experiment.
Since PPO was present in the potato we thought that the crickets that ate the potato would show the same enzyme in their bodies, however this was not the case. This actually makes sense because enzymes are made out of amino acids, which are proteins, and proteins are digested by the cricket in the midgut in the same way that carbohydrates are digested (Silva, 1996). This means that PPO would have been broken down by the cricket and would not have shown up in our tests. Therefore enzymes present in the food that crickets consume do not affect those present in its body. Further testing could be done to determine if different enzymes are needed by a cricket to digest different proteins (Gatehouse et. al., 1997).
Our data does not support the fact that the diet of a cricket determines its enzyme activity, carbohydrate content, or pigmentation. Based on the results of our tests there appeared to be no correlation between the chemical composition of a cricket and that of its food. This tells us that in the multimillion dollar industry of cricket farming there is a less expensive way to raise crickets. Potatoes and oats are very inexpensive and from our results it seems that the cricket farmers can use these food items instead of the expensive cricket food without sacrificing the nutrition value of their crickets.
This is still a topic of research that can be explored further using different tests and testing methods. There might be some difference in the crickets overall health depending on what foods it is fed. Crickets only tend to live a few weeks, so another experiment would be to test the duration of life of crickets that eat different foods.
![]()
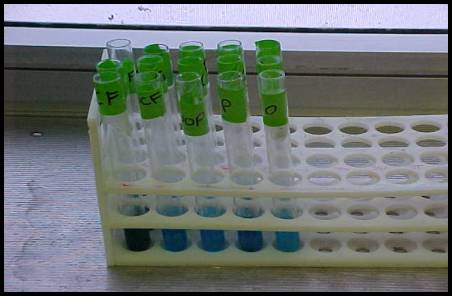
Figure 1. Barfoed's Test. Barfoed's tests for reducing sugars that are monosaccharides. Only monosaccharides will reduce copper and change color due to formation of orange/red precipitate. The cricket food (CF) was the only solution to form an orange/red precipitate, meaning that it is the only solution which contains a monosaccharide sugar. The Cricket with cricket food (CwCF) solution, Cricket w Oats and Potatoes (CwOP) solution, the potato solution (P), and the oatmeal solution (O), all remained a blue color and negative for the presence of monosaccharides.
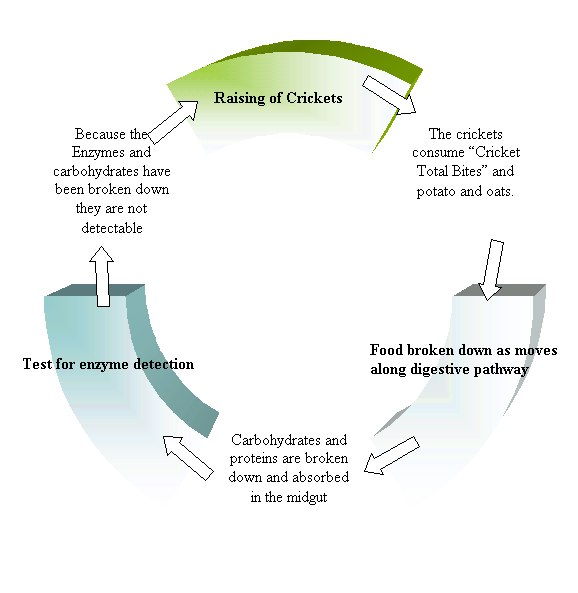

Figure 7. Enzyme and Carbohydrate absorption and digestion. This figure illustrates the possibility of why some carbohydrates and enzymes were undetectable in the crickets that consumed the cricket food and those that were on the potato and oatmeal diet. The sugars that were in the different cricket foods did enter the bodies of the crickets, but when they did they were broken down and absorbed by the cricket. Enzymes are made out of amino acids, which are proteins, and proteins are digested by the cricket in the midgut in the same way that carbohydrates are digested. This means that PPO would have been broken down by the cricket and would not have shown up in our tests.
