Barfoed’s, Iodine, and PPO Tests Show Similarities between Organic
and Processed Potato (Solanum tuberosum)
Chips
By: Team N.E.R.D.
Kendra Snyder
Jennifer Forgach
Brittany Dugger
Paul Bucchi
------------------------------------------------------------------------------------------------------------------------
Abstract
Our experiment compared organic and processed potato chips with pure potatoes, in terms of carbohydrate, pigment, and enzyme characteristics. Pure potatoes served as our main control. We hypothesized the characteristics of organic potato chips would more closely resemble those of pure potatoes than the characteristics of processed potato chips.
The carbohydrate experimental results did not support our hypothesis because they did not show any clear difference between the three variables. Barfoed’s Test showed none of the samples contained a monosaccharide and the Iodine Test for Coiled Polysaccharides showed all three contained starch.
The photosynthesis part of our experiment also did not support our hypothesis. The Pigment Separation Test showed none of the samples contained the pigments carotene, xanthophyll, chlorophyll a, or chlorophyll b. Therefore, results from the Spectrum Analysis Test did not have direct relevance to the focus of our experiment.
The enzyme analysis also did not render supportive results. The Presence of PPO Test showed only the pure potato samples contained the enzyme polyphenoloxidase (PPO). These results were reiterated by the Concentration of PPO Test which showed clearly that only the pure potato samples had a measurable concentration of PPO.
From the results of these assays, specifically the Barfoed’s Test, the Iodine Test for Coiled Polysaccharides, the Presence of PPO Test, and the Concentration of PPO Test, we found that our original hypothesis is unsupportable. We concluded that the characteristics of organic potato chips and processed potato chips actually resemble each other more than they do pure potatoes.
Discussion
Throughout this lab, we predicted that the make-up of organic potato chips is closer to that of pure potatoes than processed potato chips. After completing a series of tests, including carbohydrate, photosynthesis and enzyme analysis, our results did not support this hypothesis. The tests we performed were the Barfoed’s Test, the Iodine Test for Coiled Polysaccharides, the Pigment Separation Test, the Absorption Spectrum Test, the Presence of PPO Test, and the Concentration of PPO Test.
We started our experiment with the Barfoed’s Test. This test was performed to determine the presence of a monosaccharide among the three samples. Monosaccharides are simple sugars that are used to provide an organism with energy. Our results showed that there were no monosaccharides in any of the variables (Table 1). We concluded this because no red precipitate formed in any of the test tubes (Figure 1). A dark, sludgy precipitate which formed in the pure potato extract complicated our results. This precipitate continued to form no matter what methodological changes were made. Because it was not red, and hence was not consistent with our positive control, we regarded it as negative for the presence of a monosaccharide. The results for this test were inconclusive in regards to our hypothesis because the results for all the different samples were the same and it gave us no insight into the differences between the three.
We then performed the Iodine Test for Coiled Polysaccharides. This test was intended to distinguish the presence of the coiled polysaccharide starch in the three variables. At first, this test indicated that starch was present only in the organic chip and processed chip solutions (Table 2). We deciphered this because only these solutions formed a blueish-purple-black color precipitate (Figure 3). We found it strange that we didn’t find starch in the pure potato solution, so we decided we had to troubleshoot this part of the experiment. We thought the problem may have been because the starch was primarily contained in a part of the pure potato that was removed by the cheesecloth and hence there was not a great concentration of it in the extract we used. To test this idea, we redid our positive controls for this experiment, but this time we diluted the 1% starch solution to the different concentrations discussed in the methods. We found that even solutions with low concentrations of starch reacted to form a blueish-purple-black color precipitate just like the positive control (Figure 4). From this, we concluded that a solution with even a very low starch concentration will form the precipitate that occurred in the positive control (Table 3). Because a low concentration of starch didn’t seem to be the source of our error, we decided we had to try alternating another aspect of our experiments procedure. To accomplish this, we changed the way we obtained our extracts as outlined in our methods. When we did this, we got the results we expected. All the solutions formed a blueish-purple-black color precipitate consistent with the positive control (Figure 5). We concluded that all the solutions contained starch (Table 4). Because starch is the major form of stored carbohydrates in plants, we expected that all the variables would contain this molecule to some extent, as they did (Freeman, 130).
The results of the Iodine Test for Coiled Polysaccharides also further contributed to our conclusion regarding the samples made from the Barfoed’s Test. Starch is a polysaccharide, meaning it has many sugars which are bonded to each other through dehydration reactions. Two monosaccharides join together when their hydrated carbons react and water is released (Luckie et al, 63). A monosaccharide does not have these dehydrated bonds, so it is considered a single sugar. Starch was contained in all the variables, and since it is a polysaccharide, it makes sense that no monosaccharides were present when we performed the Barfoed’s Test.
To determine the pigments and their rate of flow, we used the Pigment Separation Test. Our original hypothesis was that processed potato chips would contain added pigments. The definition of a pigment is a substance that absorbs visible light (Luckie et al, 12). We were primarily looking for photosynthetic pigments. The pigments we tested for using paper chromatography were carotene, xanthophyll, chlorophyll a, and chlorophyll b. These pigments, called antenna pigments, are located in the thylakoid membrane of the chloroplasts (Luckie et al, 10). Potatoes are tubers; enlarged roots which absorb water and nutrients from the soil and act as a storage place for starch (Freeman 605). They do not need pigments to absorb light, because they don’t perform photosynthesis (Freeman, 605). This explains why we did not find any of the photosynthetic pigments we were testing for in the potato based samples (Figure 6). Contrary to what we found, past experimentation dealing with pigments in potatoes has found that the pigments called carotenes are present (Forster et al). This contradiction may be due to different procedure we used for preparing the solutions we tested.
Another pigment analysis test we used was the Absorption Spectrum Test. This test again concentrated on the pigments of our samples. In particular, this test was meant to deal with the absorbencies of the photosynthetic pigments we had expected to separate in the Pigment Separation Test. Like the Pigment Separation Test, this test did not result in the data we expected (Table 5). When we graphed the average absorbencies of the three trials, there was no clear pattern to the graph and we could not conclude anything from it (Figure 7).
Our last tests dealt with the enzyme polyphenoloxidase (PPO), which is known to be found in large quantities in potatoes. The first test we performed, the Presence of PPO Test, was intended to determine whether or not the enzyme was present. The pH, indicated by the color of the litmus strip, was slightly lower for the organic and processed potato chips than the pure potato slice. From the color change of the pure potato slice to a rusty brown when catechol was added, and the lack of color change in the other samples, we concluded that there is only PPO present in the pure potato samples (Figure 8) (Table 6). We believe the reason PPO was not found in the processed and organic potato chips is due to their cooking process and the fact they are more acidic than pure potatoes. All enzymes have a certain pH at which they function optimally, and the more acidic environment in the two types of chips may have inhibited the function of PPO. Enzymes also can be affected by heat. In class-room experimentation, when PPO was boiled and 350μl of 0.1% catechol solution was added, no color change occurred because the exposure to heat had inhibited the enzymes function. Potato chips are cooked at temperature well over 100º Celsius, and this heat is more than enough to affect the enzyme PPO (Wilson, 2002).
The last test in our experiment, the Concentration of PPO Test, was intended to compare the concentration of PPO in our three samples in relation to each other. However, in the Presence of PPO Test, we found that only pure potatoes contained any PPO, so no such comparison could be made. The slope of the linear change in absorbency for both the processed and organic potato chips was nearly 0, meaning the absorbency did not change at all because PPO was either not present or not functioning properly (Figure 9). The absorbency of the pure potato solution changed over time because the PPO reacted with catechol making the solution darker, and hence changing its absorbency (Table 7). Once again, the results of this test did not support our initial hypothesis.
We set out to complete this experiment hoping to illustrate that there are more similarities between pure potatoes and organic potato chips than processed potato chips. We used the pure potato as our control and compared the other two samples to it. Through this series of tests we found that the carbohydrates, photosynthetic pigments, and enzymes we tested in the organic potato chips were not more similar to the pure potatoes than the processed potato chips. We actually found support for the contrary. The characteristics of the organic and processed potato chips more closely resembled each other than the pure potatoes. Most of our findings, we believe, can be attributed to the cooking process and are comparable to other published studies.
Water loss is a major tradeoff when processing the potato into potato chips. Potato chips are 1.8% water which is only 75% of the amount in a pure potato (indiapotatoes). They also have 33 more grams of carbohydrates than a pure potato in addition to more calcium, iron, ascorbic acid, thiamine, riboflavin, and niacin (indiapotatoes). This noted increase in the amount of carbohydrates and nutrients may be due to the cooking methods of the potato chips which include frying the potato slices in oil. Companies may also add ingredients to intensify the flavor. These two events may account for the loss of nutritional factors.
The observed differences may have occurred by chance or are due to some other cause we are not taking into account. Also, the problems we ran into with developing a procedure that yielded for us an extract that could be used in the experiments, may be scrutinized. Some may say that we manipulated this procedure to get the experimental results we expected, but this is not the case. We adjusted this procedure because the experimental results we were getting were not consistent with factual data, like the presence of starch in potatoes, which is accepted truth throughout the scientific community. Others could also question the brand of potato products we chose to test and even the source of the pure potatoes we used. These are all noteworthy critiques, but are not really relevant for the basic level of the experimentation we performed.
There are many possible sources of error in our experiment. Results could have varied due to inconsistent measurements, faulty equipment, and human error. In addition to these potential sources of error, cleanliness may have played a factor as well. Any unknown contaminant may have an adverse reaction on our data. If we were to conduct this experiment again, we would double check that all of our glassware and equipment was clean and make new solutions upon every sequence of experimentation to avoid any chemical changes from storage time.
After hypothesizing and carrying out this experiment, several new questions can be raised. Our data actually showed that processed potato chips and organic potato chips more closely resemble each other than they do pure potatoes. This is an interesting point to think about. Potato chips start off as pure potatoes which alone are very nutritional. However, as they are made they lose their nutritional value. This means that the best of both worlds, taste and nutrition, could be reached if somehow the nutritional value of potato chips could reestablished at the level of pure potatoes after processing. After researching this issue we realized that the current attempts to make potato chips more-healthy, for example, the production of organic chips, have not worked. Organic potato chips and processed potato chips are very much the same. No matter what the companies claim, or how much more they are able to charge you for the organic potato chips, a lot of work still needs to be done to make potato chips that have the same nutritional value as pure potatoes.
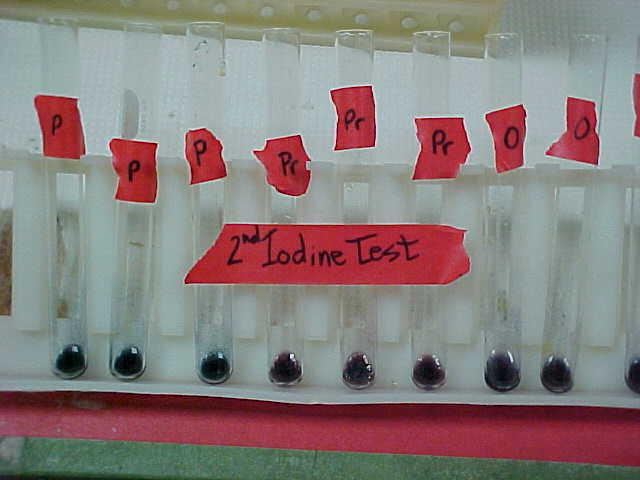
Figure 5: The Iodine
Test for Coiled Polysaccharides #2 Results. This is a picture taken of our
results from the Iodine Test for Coiled Polysaccharides, which tests for the
presence of coiled polysaccharides including starch, from the second time we
completed this portion of our experiment with the new and differently prepared
sample solutions. Each test tube is labeled with a letter that stands for the
sample solution type. “P” stands for pure potato, “O” for organic chip, and
“Pr” stands for processed chip. If a coiled polysaccharide is present in the
tested solution, a bluish-purple-black color precipitate will form in the
solution, which has occurred for all our sample solutions. From this, we
concluded that all our samples contain starch and that something in the way we
had produced our first set of sample solutions had thrown off our results.