The Buffers
(Jaime Engelhart, Cara DeSanto, Lauren Walker, & Chas Pudrith)





Here are our theories on...
A Comparison Citrus sinensis Rind & Flesh By
Quantitative Analysis
Abstract:
Our intention in this lab was
to prove the idea that the rind of the orange may contain nutrients, but not in
a substantial form when compared to the flesh. This was done with a sugar, photosynthetic, and enzyme lab.
The
sugar lab included Benedict’s, Barfoed’s, Selavinoff’s, Bial’s and Iodine
tests. The results indicated the presence of aldoses and a lack of
starches. The results also
indicated that the flesh had aldehydes and the rinds did not. Monsaccharides were also found only in
the rind while only the flesh had furanose rings. This proved that while the rind had some sugars they were
not of substantial value.
The photosynthetic lab included a paper
chromatography and an absorption spectrum test. The paper test indicated that both the flesh and the rind
had an Rf value of 1. This
indicated the presence of xanthophyll.
With the spectrophotometer, we measured the absorbencies of the rind and
the flesh at various wavelengths and received no information.
The enzyme lab included a pH
test, 2 types of catechol tests, and a taste test. The flesh had a higher pH than the rind. The first test measured the change in
absorbency. The second test was
for a color change when placed directly on the flesh and rind. Both of those test came back negative
implying the lack of PPO and we were unable to conclude anything from these
tests. In the taste test, the flesh received 3 of 4 stars and the rind received
only 2.
Figures:
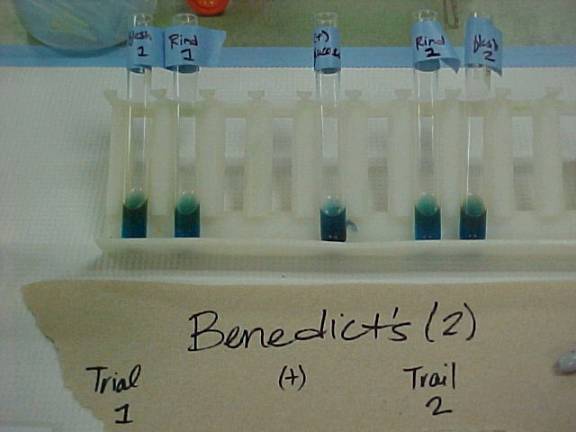
This is a picture of our results from the Benedicts test, the first of 5 sugar
tests. (From left: Flesh 1, Rind 1, Positive Glucose
Control, Rind 2, Flesh 2) Although
it is difficult to notice, there is a red copper precipitate that has formed in
the two flesh test tubes and the positive control. Although a reaction did occur with the rind solution, there
still was not a copper precipitate, therefore the rind tested negative.
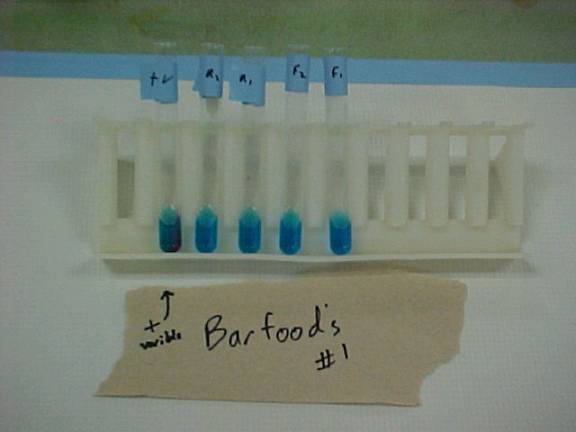
This is a picture of Barfoed’s test. (From left: Positive Fructose Control, Rind 1, Rind 2, Flesh 1, Flesh
2) In this test determined what
saccarides were present within each orange part. The color change in the positive control can be easily seen,
but the rest of the results are not.
The flesh did not react, but the rind did have a slight color change,
which renders a positive result, it is a monosaccaride.
* Benedict’s and Barfoed’s Assays shown in the above pictures. These are the most relevant figures we found for our experiment. This is because from information researched, more energy is given off over time in the flesh since it contained polysaccharides as shown in the results of these tests; therefore, the flesh of the orange has proven to be more nutritious than the rind. *
Discussion:
We based our experiment on the simple question: Does the rind
contain any nutritional value? Once
this question was asked a secondary question followed: If it does have some
nutritional value how does it compare to the flesh of the orange? Should people
continue to throw out the rind of the orange without any thought? We hypothesized that while the rind of
the orange may have some trace amounts of nutritional value, there would be no
substantial benefit of eating the entire orange, rind included. After careful experimentation we were
able to confirm our hypothesis finding that the nutritional value in the orange
was too small to be of substantial value when compared to the flesh.
The
first set of experiments we conducted were to investigate the presence of
specific carbohydrates and their traits. The basis and structure of this lab
closely follows the procedures found in our Laboratory Course Pack. If you refer to Table 1, you will see
the experiments performed and each experiment’s results for the flesh, the rind
and our positive control. Stock
solutions of both the rind and the flesh were blended daily and can be seen in
Figure 1. In each test a given
substance was also tested on, this particular carbohydrate had been tested
prior to the lab and known to have a positive result. This allowed us a standard to hold our data up to, also
indicating that the test was performed properly. The first test we used Benedict’s reagent. This is used to determine if there are
free aldehyde or ketone groups. We
tested both the flesh and the rind twice and found that for each of the two
flesh samples came out positive while the rind samples were both negative. The flesh’s positive results indicated
the presence of reducing sugars.
This is indicated in Figure 2 where a red precipitate can be seen. The reduction of carbohydrates is very
important. If a chain cannot be
easily broken down and digested, it cannot be used as a source for energy. The results of Benedict’s Reagent
helped to confirm our hypothesis when the flesh tested positive for the
reducing sugars.
The next test was executed using Barfoed’s solution, which
indicated the presence of monosaccharides, disaccharides, and
polysaccharides. In the flesh of
the orange the test both of the results came back negative, while the rind
samples tested positive for monosaccharides. The results of this test where there is a slight color
change can be seen in Figure 3.
The presence of the monosaccharides in the rind leads to the assumption
that only simple carbohydrate are to be found in the rind. A diet less in simple carbohydrates is
recommended since they can be quickly broken with very few lasting
benefits. Barfoed’s solution also
supported our hypothesis that the nutritional value found in the rind is
insignificant when compared to the flesh.
Then we used Selivanoff’s reagent to see whether or not these two
parts contained ketoses and aldoses.
Since both the rind and flesh samples resulted in a positive test for
the presence of aldoses we were unable to conclude which one might have been
healthier. These results can be
seen in Figure 4. With Bial’s we
were able to determine if furanoses were present. The flesh samples came out negative while the rind samples
were both positive. We were not expecting results like this. The five carbon ring, commonly referred
to as furanose, was found only in the rind and is an important contributor in
the production of energy. The
slight variation in color can be noted in Figure 5. These results negate our hypothesis. We were unable to find the presence of
any starches in either the rind or the pulp. Since the both came back negative, our results were
inconclusive for this particular test.
We also used Iodine to test for the presence of starches but both tests
for the rind and the flesh came back negative and we were unable to conclude
anything about our hypothesis based on starches alone. The result of these tests can be viewed
in Figure 6.
The second major component we tested our hypothesis with was for the presence of chlorophyll , which is a positive indication for the process of photosynthesis. “This is a very important process and is essential for the existence of life. The formation and maintenance of any living system requires compounds that were formed by transforming: (i) light energy to chemical energy, and (ii) inorganic carbon to organic carbon.” (Course Pack, 71). First we used a chromatogram strip as a way to identify pigments found in the orange and rind. Please refer to Figure 7 to see the results. We were unsuccessful in finding an orange leaf in which we had hoped to compare the levels of chlorophyll a and b and trace the decreasing levels from the leaf to the rind to the flesh. But when the flesh and the rind pigments were compared alone chlorophyll a and b were not found in either. We were only able to conclude that an RH factor of 1 was found both times in each the rind and the flesh. This indicated the presence of only one pigment and that pigment was xanthophyll and is shown in Table 2. Since the oranges we used were of the ripe variety we concluded that since it had reached optimal size the photosynthesis process was no longer necessary in the maturation of the orange. At different stages in growth of the orange it is obvious that chlorophyll is present because the rind has green pigments. Since energy is required to keep producing sugars to add to the growth of the orange photosynthesis creates chlorophyll. This process stops and the presence of the pigments disappears once the orange has matured. Since there was no presence of these two important pigments our results were inconclusive.
A separate test we performed also looking at the important process
of photosynthesis was for the absorption spectrum of the orange vs. its rind.
The levels we found using the spectroscope demonstrated no assistance in
helping us compare the nutritional value of the orange and the rind. This can be seen in both Figures
7 and 8. Since both of our samples do not contain chlorophyll this test was
unable to support or negate or hypothesis. If we could have done this lab again we would have ordered
orange leaves from an orchard or nursery allowing us to verify that
photosynthesis is indeed used in the production of the fruit. We would have also liked to have been
able to test for other possible pigments that might trace the chlorophyll
better from the leaf to the rind to the flesh.
In
our last section we tested for the presence of the enzyme PPO in both the rind
and the flesh. PPO is a enzyme
commonly found in fruit that acts as a catalyst and is responsible for giving
fruit that brownish color when cut and exposed. (Course Pack, 80) The first thing we tested was the pH
level, we found that the pH of the flesh was about 4 indicated that it was
acidic. The rind was also slightly
acidic but only had a pH level of 6. If you look at Figure 10 you can see our results. From this alone we were unable to
conclude anything that related to our original hypothesis. When a slice or the
flesh and rind were coated with 2 drops of catechol solution and monitored over
time there was no change in the color of the orange. This was the first indication that PPO was not found in
either the orange or the rind.
Figure 11 shows the lack of any color change in both the rind and flesh
sample. We decided to do a
second separate test to insure that PPO was not present. When the both the rind and the flesh
were compared using the spectrophotometer. Solutions of both the rind and the flesh were each mixed
with water and catechol. The
transmittance readings of each one were then taken at one-minute
intervals. These results can
be viewed in either Table 4 or Figure 12.
Since there was more of a change in the water we concluded that PPO was
not found in either the orange or the rind and that the small variance in the
transmittance levels was a result of mechanical error. If we were to have had more time and
better equipment we would have like to look that transmittance levels of the
rind and the orange over a greater span of time under much more detail. Chas volunteered himself to do the
taste test. We decided taste is an
important factor in the consumption of food. The taste of the rind explained as somewhat neutral and
bland. Not great, but no horrible
either. Chas’s 4 star rating
system can be seen in Table 5.
Throughout
the course of the lab we ran into several difficulties. Our most important
findings were from the first set of experiments where we found the presence of
different sugars. With the other
sets of experiments it was harder to collect information that would help us
either negate or support our hypothesis. Could we have repeated this test
knowing what we now know there would be several things we would like to
change. When precipitate were
formed in specific tests we should have found ways to measure the amount of
each and compared the two levels.
Quantitative analysis should have been compared as well as
qualitative. Perhaps if we would
have placed the test tubes with precipitates in the centrifuge and then used
the spectrophotometer we would have been able to have quantitative data as well
as qualitative. We were able to
learn a great deal of information both about oranges as well as methods used to
test in a laboratory setting. Through our mistakes we were able to take away a
lesson learned and a better approach to our next similar situation. We would
have like to have performed other tests in which other nutritional factors
like, vitamins, could have been compared.
There were probably numerous human errors that took place on our part
that might have had an effect on our overall results of the experiment. The small amount of lab experience we
had between the four of us was obvious at many points in time. But we believe that our information we
found and provided is correct and using the information we were able to reach a
conclusion. We found that the
experiments we conducted supported our original hypothesis that although the
rind may contain some nutritional value it is insignificant when compared to
the flesh and would not be worth consuming.
J We hope everyone
enjoys our website & have learned something from it!! Have a nice day! J





Email Us: